Fanapt® demonstrated efficacy with placebo-like rates of akathisia1
Percentage of Fanapt®-treated patients who experienced
drug-induced akathisia at both high and low doses was comparable to
placebo1

Percentage of Fanapt®-treated patients who experienced
drug-induced EPS at both high and low doses was comparable to
placebo1
- 13.5% of subjects taking Fanapt® (iloperidone) 10 to 16 mg/day and 15.1% taking 20 to 24 mg/day experienced an EPS event compared to 11.6% taking placebo1
- Akathisia and EPS data from 4 placebo-controlled, 4- or 6-week, fixed- or flexible-dose studies1
- No significant difference was observed between placebo- and Fanapt®-treated groups
In a separate pooled analysis of longer-term trials (4 open-label
extensions, 3 double-blind studies) with a duration of up to 12
months, incidences of akathisia adverse events were below 2%2*†‡
- *Longer-term trials consisted of 4 open-label extensions and 3 double-blind, 52-week studies. Results were recorded in the following time periods: >6 weeks to 3 months (N=1822), >3 months to 6 months (N=1427), >6 months to 12 months (N=1172).
- †Drug-induced adverse events were defined as those that were new or aggravated in severity or frequency after administration of the first dose of study drug through 3 days after the last dose of study drug.
- ‡In the placebo-controlled studies, patients in the placebo group received treatment for up to 4 or 6 weeks; therefore, no data are reported.
EPS = extrapyramidal symptoms.
Learn more about safety and tolerability
Weight gain
Weight gain is a concern with atypical antipsychotic therapy
In clinical trials, the majority of Fanapt®-treated patients did not experience clinically significant weight gain1
- 12% (taking 10 to 16 mg/day) and 18% (taking 20 to 24 mg/day) of Fanapt®-treated patients experienced clinically significant weight gain (≥7% increase in body weight from baseline) vs 4% for placebo§
- Average weight gain across all short- and long-term studies for Fanapt®-treated patients was 2.1 kg (4.6 pounds)
- §Based on pooled results from 4 placebo-controlled, 4- or 6-week, fixed- or flexible-dose studies in adult subjects (N=1448).

Fasting lipids and blood glucose
Metabolic changes are a concern with atypical antipsychotic
therapy
In clinical trials, the majority of Fanapt®-treated patients did not experience clinically significant shifts in fasting lipids and blood glucose1
Patients experiencing shifts from normal to high1,a-c
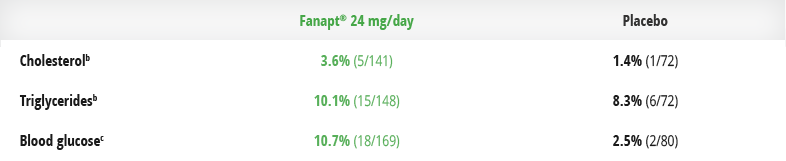
- aBased on data from a placebo-controlled, 4-week, fixed-dose study.
- bShift from <200 to ≥240 mg/dL in fasting total cholesterol. Shift from <150 mg/dL to ≥200 mg/dL in fasting triglycerides.
- cShift from <100 to ≥126 mg/dL.
Common adverse events
Fanapt® discontinuation rates due to adverse events were similar to placebo in pooled data from 4 short-term studies1II

Most common adverse reactions (incidence ≥5% and 2-fold
greater than placebo for at least 1 dose) in short-term (4- to
6-week) studies1

- Fanapt® prolongs QT interval and may be associated with arrhythmia and sudden death. Avoid use of Fanapt® in combination with other drugs that are known to prolong QTc; use caution and consider dose modification when prescribing Fanapt® with other drugs that inhibit Fanapt® metabolism. Monitor serum potassium and magnesium in patients at risk for electrolyte disturbances
As with other drugs that antagonize dopamine D2 receptors,
Fanapt® elevates prolactin levels. Lower elevations
of prolactin were observed with Fanapt® than with
some other antipsychotic medications1II
- Gynecomastia (n=2) and galactorrhea (n=8) were reported in 10 of 3210 adults treated with Fanapt®, based on pooled results from clinical trials, including longer-term trials
- IIBased on pooled data from 4 placebo-controlled, 4- or 6-week, fixed- or flexible-dose studies.
Patients may achieve an efficacious dose in as few as 4 days.1
Find out more
Find out more
References: 1. Fanapt®
package insert. Washington, DC: Vanda Pharmaceuticals, Inc.;
04/2024. 2. Data on File, Vanda Pharmaceuticals,
Inc.