Fanapt® significantly improved overall schizophrenia symptoms as measured by PANSS1,2
Improvement in symptoms in a 4-week study2
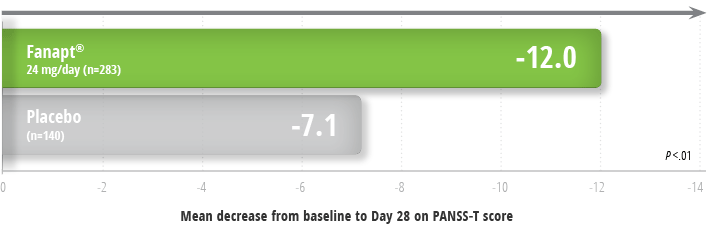
- In this study, Fanapt® (iloperidone) demonstrated similar efficacy to ziprasidone, which also required a slow titration to the target dose1
Study design: 4-week, placebo- and
active-controlled trial (n=604) involved one fixed dose of
Fanapt® (24 mg/day) compared to placebo- and an
active-control (ziprasidone). The titration was similar to that of
the 6-week study. Fanapt® was titrated at 1 mg twice
daily on Day 1, and the dose was increased to 2, 4, 6, 8, 10, and 12
mg twice daily on Days 2, 3, 4, 5, 6, and 7, respectively. The
primary endpoint was change from baseline on the PANSS total score
at the end of treatment (Day 28) analyzed using mixed-effects model
repeated measures (MMRM). Mean baseline scores were 92.88 for
Fanapt® and 90.48 for placebo.1,3
PANSS-T = Positive and Negative Syndrome Scale total score.
Fanapt® significantly improved overall schizophrenia symptoms as measured by BPRS1,3
Improvement in symptoms in a 6-week study3
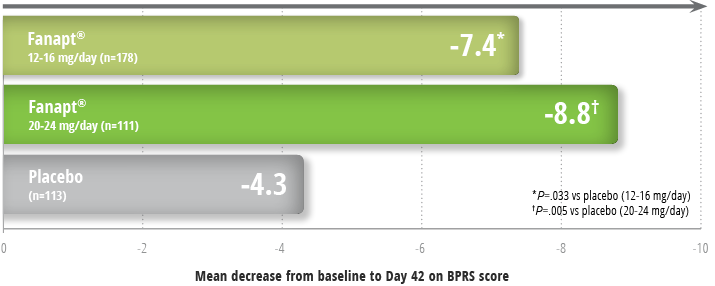
Study design: 6-week, placebo- and
active-controlled trial (n=706) involving 2 flexible dose ranges of
Fanapt® (12 to 16 mg/day or 20 to 24 mg/day) compared to
placebo and an active-control (risperidone). Fanapt® was
titrated to the respective dose over a period of 7 days. The primary
endpoint was change from baseline on the BPRS total score at the end
of treatment (Day 42).1
Patients taking Fanapt® at both high and low doses
demonstrated significant reductions from baseline in BPRS score at
Day 421:
- The active-control antipsychotic drug (risperidone) appeared to be superior to Fanapt® in this trial within the first 2 weeks, a finding that may in part be explained by the more rapid titration that was possible for that drug. In patients in this study who remained on treatment for at least 2 weeks, iloperidone appeared to have had comparable efficacy to the active control (risperidone)1
BPRS = Brief Psychiatric Rating Scale;
Fanapt® Demonstrated Long-Term Efficacy by Delaying Time to Relapse1
KAPLAN MEIER ESTIMATION OF PERCENT RELAPSE/IMPENDING RELAPSE FOR
FANAPT® AND PLACEBO-TREATED PATIENTS1*
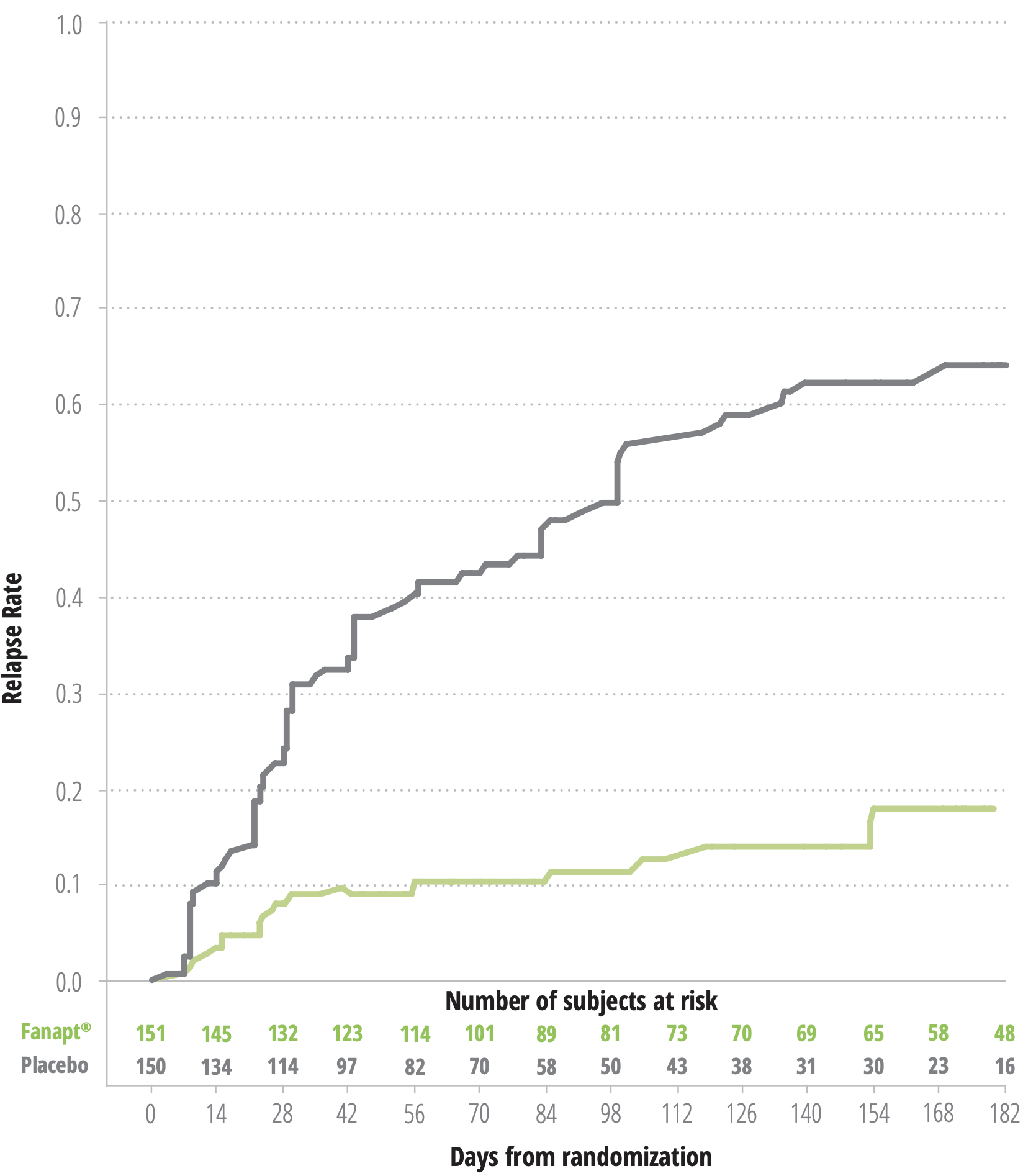
(log rank test: P<.0001)
*Based on final analysis dataset.
Study design: A double-blind, placebo-controlled,
randomized withdrawal study (n=303) involving a flexible-dose range
of Fanapt® (8-24 mg/day) administered as twice-daily
doses. The study consisted of a 7-day titration period where
Fanapt® was titrated at 1 mg twice daily on day 1, 2 mg
twice daily on day 2, 4 mg twice daily on day 3, 6 mg twice daily on
day 4, 5, 6 and 7, followed by a stabilization period lasting at
least 14 weeks. During the subsequent double-blind relapse
prevention phase lasting up to 26 weeks, patients were randomized
to placebo or established dose of Fanapt® and observed
for relapse or impending relapse. Stabilization was defined as
being on an established dose of Fanapt® that was
unchanged due to efficacy in the 4 weeks prior to randomization,
having a CGI-Severity score ≤4 and PANSS score ≤70, a score
of ≤4 on each of the following individual PANSS items
(P1-delusions, P2-conceptual disorganization, P3-hallucinatory
behavior, P6-suspiciousness/persecution, P7-hostility, or G8-
uncooperativeness), and no hospitalization or increase in level of
care to treat exacerbations. The primary endpoint was time to
relapse or impending relapse compared to placebo, defined as any
of the following: hospitalization due to worsening of
schizophrenia; increase (worsening) of PANSS score of ≥30%;
CGI-Improvement score ≥6; patient had suicidal, homicidal, or
aggressive behavior; or need for any other antipsychotic
medication, including increase in study medication1,4.
CGI, Clinical Global Impression.
CGI, Clinical Global Impression.
- BASED ON THE INTERIM ANALYSIS, THE MEAN TIME TO RELAPSE WAS 139 DAYS FOR FANAPT® VS 71 DAYS FOR PLACEBO4
- AFTER 6 MONTHS, 79.6% OF PATIENTS TAKING FANAPT® DID NOT RELAPSE VS 36.6% ON PLACEBO, BASED ON THE INTERIM ANALYSIS4
- NO NEW SAFETY SIGNALS COMPARED TO THOSE OBSERVED IN SHORT-TERM STUDIES4
- FLEXIBLE DOSING OF FANAPT® (8-24 MG/DAY) WAS EFFECTIVE1
Fanapt® demonstrated efficacy with placebo-like rates
of akathisia.1
Find out more
Find out more
References: 1. Fanapt®
package insert. Washington, DC: Vanda Pharmaceuticals, Inc.;
04/2024. 2. Cutler AJ, Kalahi AH, Weiden PJ,
Hamilton J, Wolfgang CD. Four-week, double-blind, placebo- and
ziprasidone-controlled trial of iloperidone in patients with acute
exacerbations of schizophrenia. J Clin Psychopharmacol.
2008;28(2 Suppl 1):S20-S28. 3. Data on File, Vanda
Pharmaceuticals, Inc. 4. Weiden PJ, Manning R, Wolfgang CD, et al. A randomized trial of iloperidone for prevention of relapse in schizophrenia: the REPRIEVE study. CNS Drugs. 2016;30(8):735-747.