Fanapt® demonstrated efficacy with rates of akathisia that were low or similar to placebo1,2,✝︎
Percentage of patients with worsened Barnes Akathisia Rating Scale
global scores from baseline2

- *Patients with poor CYP2D6 metabolizer status received 12 mg/day (n=15/206 in the complete Fanapt® study population)
- ✝︎Barnes Akathisia Rating Scale results in graph represent results for the observed cases at baseline and Day 28
Percentage of Fanapt®-treated patients with akathisia or
extrapyramidal disorder was low or similar to placebo1,2
- In the short-term studies in schizophrenia, rates of akathisia in patients taking Fanapt® were 2.3% and 2.7% for placebo.
- In the single bipolar I study, the rate of akathisia in Fanapt® patients was 4.4% and 0% for placebo.
Learn more about safety and tolerability
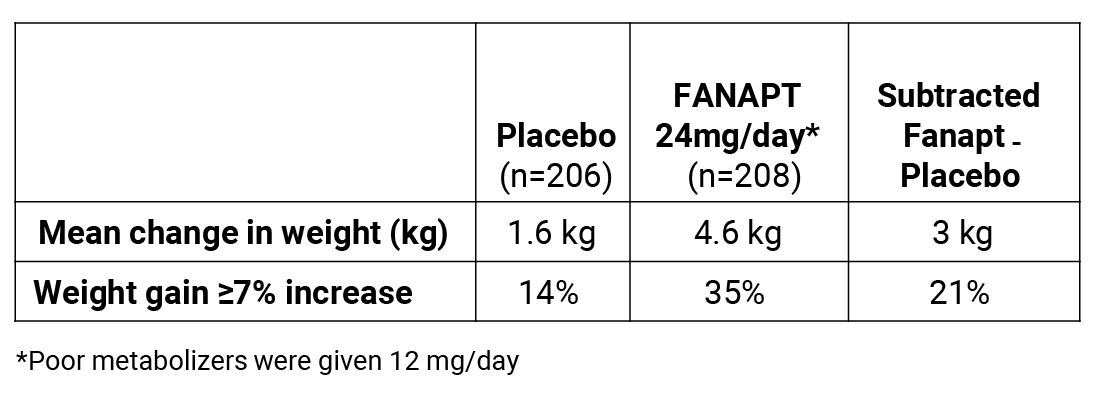
Weight gain
Weight gain is a concern with atypical antipsychotic therapy
In clinical trials, more than 65% of patients taking Fanapt® did not experience significant weight gain1
Changes in body weight (kg) and % of subjects with significant weight gain
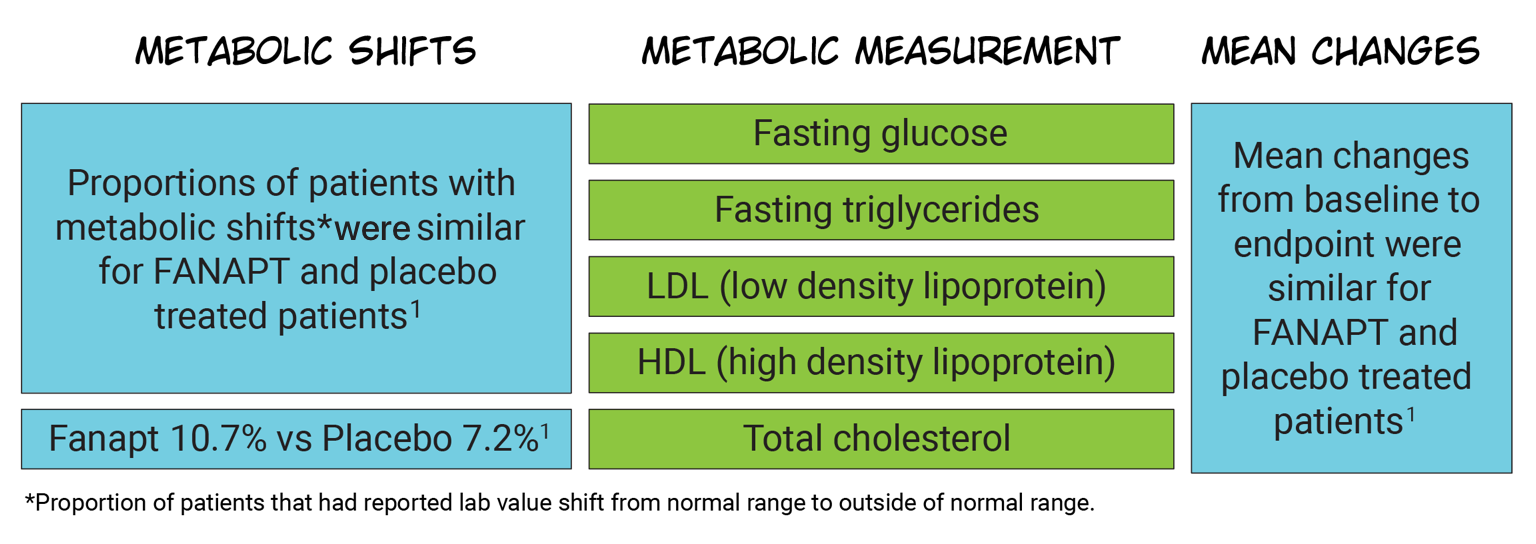
Fasting lipids and blood glucose
Metabolic changes are a concern with atypical antipsychotic
therapy
The majority of Fanapt®-treated patients in a 4-week bipolar mania study did not experience significant shifts in fasting lipids and blood glucose1
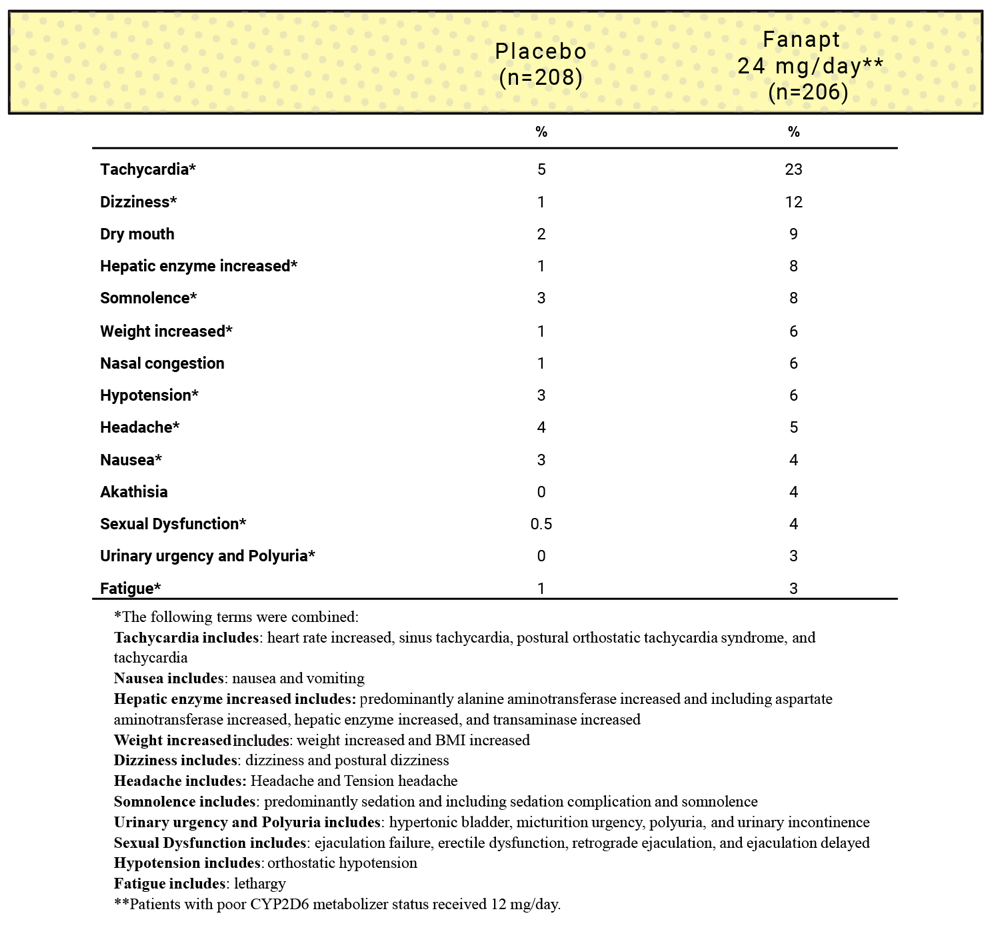
Common adverse events
Most common adverse reactions (incidence ≥2% and greater than placebo for at least 1 dose) in a short-term (4-week) study1
Patients may achieve an efficacious dose in as few as 5 days.1
Find out more
Find out more
References: 1. Fanapt®
package insert. Washington, DC: Vanda Pharmaceuticals Inc.; 04/2024.
2. Torres R, Czeisler EL, Chadwick SR, et al.
Efficacy and safety of iloperidone in bipolar mania: a double-blind,
placebo-controlled study. J Clin Psychiatry. 2024;85(1):23m14966