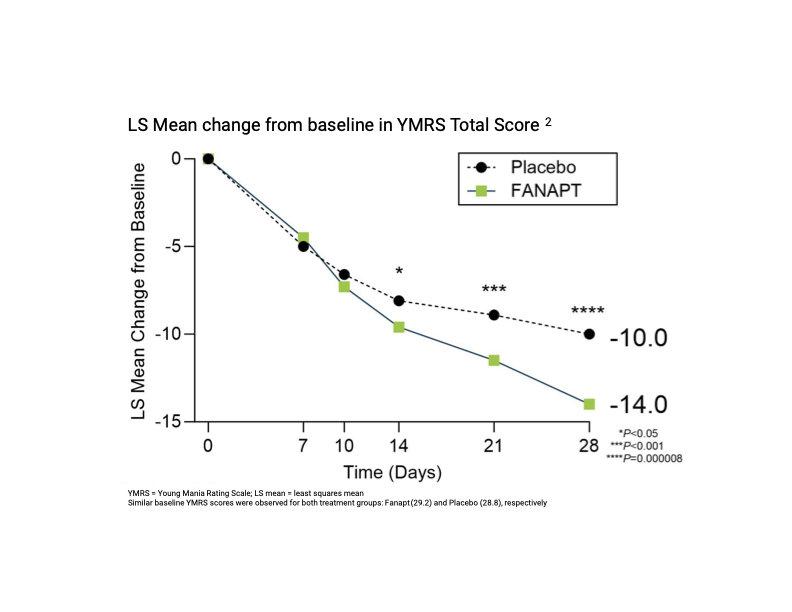
Fanapt® significantly improved overall bipolar mania symptoms in a 4-week placebo-controlled study, as measured by the YMRS1,2
IMPORTANT SAFETY INFORMATION
Neuroleptic malignant syndrome, a potentially fatal symptom, has been reported in association with antipsychotic drugs, including Fanapt®. Manage with immediate discontinuation of drug, treatment if needed, and close monitoring.
Tardive dyskinesia: The risk of tardive dyskinesia may increase as the duration of treatment and total cumulative dose increases. Discontinue Fanapt® if clinically appropriate.
Seizures: Use Fanapt® cautiously in patients with a history of seizures or with conditions that lower seizure threshold.
Falls: Fanapt® may cause somnolence, postural hypotension, motor and sensory instability, which may lead to falls causing fractures or other injuries. For patients with diseases, conditions or medications that could exacerbate these effects, complete fall risk assessments initially and recurrently during therapy.
Leukopenia, neutropenia, and agranulocytosis have been reported with antipsychotics. Patients with a pre-existing low white blood cell count (WBC) or a history of leukopenia/neutropenia should have their complete blood count (CBC) monitored frequently during the first few months of therapy and should discontinue Fanapt at the first sign of a decline in WBC in the absence of other causative factors.